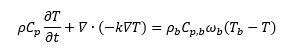Multiphysics Modeling of Cooled RF Ablation
In previous blog posts, we have provided extensive discussion on the Use of Computational Simulation of Medical Technology[1]. To provide a specific demonstration, this blog post will focus on describing the implementation of a multiphysics simulation of a cooled RF ablation (C-RFA) device.
Traditional vs Cooled RF technology
Traditional radiofrequency ablation (RFA) technology seeks to destroy unwanted tissue using radiofrequency energy introduced into the tumor through electrode probes. A major challenge of this approach is that the size of ablated lesions are limited due to excessive heating near the electrode/tissue interface that can cause desiccation and charring[2]. A significant drop in the electrical conductivity of the tissue when moisture is vaporized effectively creates an electrical barrier around the electrode, mitigating the therapeutic benefits.
C-RFA seeks to reduce peak temperatures near the electrode/tissue interface, thus reducing vaporization through forced coolant flow within the lumen of the metal electrodes. For this reason, C-RFA is able to treat larger tumors by allowing for energy to be deposited deeper and longer into the diseased organs without local, near electrode effects, affecting energy deposition.[3]
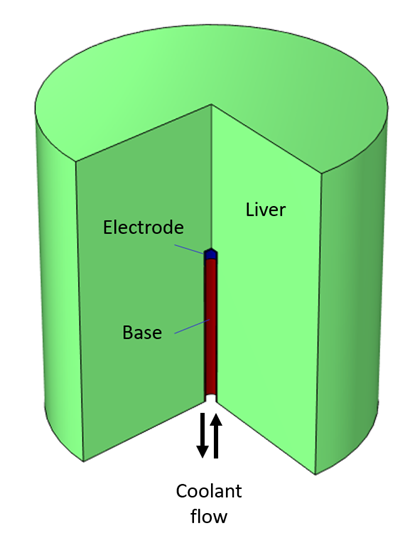
Figure 1. Electrode tip of a C-RFA probe. Cutaway view shows flow of coolant.
Multiphysics computational model
To simulate the use of C-RFA technology, a physics-based predictive simulation model has been developed using COMSOL Multiphysics. A schematic of the problem set up is shown in Figure 2.
Figure 2. Analysis geometry used to predict liver tissue ablation zone size and shape.
The standard transient heat balance equation for conduction and heat storage is implemented and supplemented with a blood perfusion within the tissue to represent typical in vivo conditions:
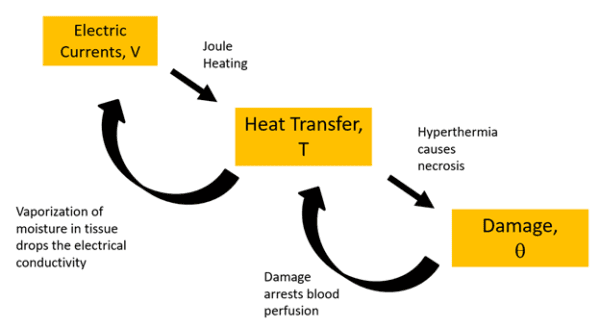
Forced convection losses due to coolant flow are represented using heat transfer coefficients based on the flow velocity and sink temperature of the inflowing coolant. Electric currents equations are solved based on Ohm’s law. A ramp function is used to represent the order of magnitude drop in electrical conductivity of the liver when its liquid vaporizes at 100°C. The temperature-time history is integrated based on an Arrhenius damage integral during the simulation enabling tracking of the size of the necrotic lesion as well as concurrent adjustment of thermal properties including the arresting of blood perfusion upon coagulation. Material properties for the thermo-electrical response of liver tissue are taken from the COMSOL Bioheat Materials Library[4]. Figure 3 represents the intimately coupled interactions between all three solution variables and physical equation sets.
Figure 3. Physics and couplings of simulation.
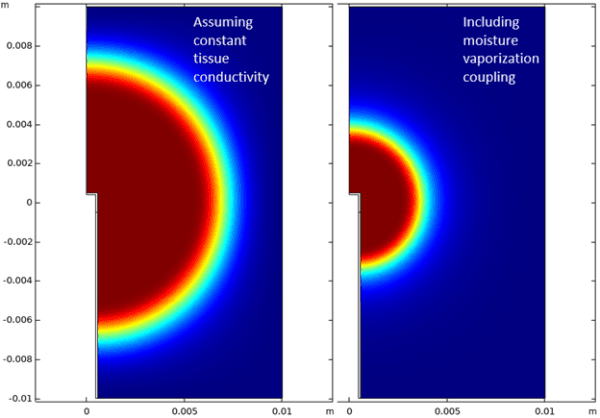
The intimately (bi-directionally) coupled set of equations are solved simultaneously for each time step using COMSOL’s fully coupled equation solvers. Figure 4 compares model results with and without the effect of vaporization included. The predicted lesion size when the loss of conductivity due to vaporization is included is approximately half the diameter, indicating that vaporization is essential for predicting accurate legion sizes.
Figure 4. Fraction of necrotic tissue after 2 minutes of RF therapy assuming constant tissue conductivity, i.e., no desiccation (LEFT) and including the moisture vaporization coupling term (RIGHT). The model predicts only half the lesion size in the 2nd case which is expected to be more accurate.
Conclusion
Based on this case study, the following observations can be made:
- The size of RF ablated lesions is highly dependent on the electrical properties near the tissue electrode interface. Therefore, numerical models of ablation should include any significant temperature and phase-dependent changes in this region including conductivity loss due to liver tissue desiccation.
- Cooled RF ablation technology mitigates vaporization and charring, reducing tissue temperatures near the electrode, and may allow the development of larger lesion sizes compared with standard RF probes.
To accurately predict RF therapy lesion size that includes the complicated thermo-electrical interaction of the probe with living tissue, an intimately coupled set of governing equations must be solved. COMSOL Multiphysics is a tool well-suited to implement and solve such equations.
This model has also been used by AltaSim to explore legion size sensitivity to design and operating parameters including coolant flow rate and temperature, electrode therapy duration/pulsing and the magnitude of the input current.
We hope that this case study shows designers and regulators of RF ablation devices that they could leverage AltaSim’ s expertise in advanced modeling techniques for proof of concept, optimization, or safety studies of their RF ablation products or other bioengineering systems.
______________________________________
[1] https://altasimtechnologies.com/use-of-computational-simulation-of-medical-technology-part-1/
[2] Pereira PL, Trubenbach J, Schenk M, Subke J, Kroeber S, Schaefer I, Remy CT Schmidt D, Brieger J, Claussen CD. Radiofrequency ablation : in vivo comparison of four commercially available devices in pig livers. Radiology 2004; 232:482-490.
[3] https://www.mycoolief.com/articles/how-does-coolief-cooled-rf-work/
[4] P.A. Hasgall, F. Di Gennaro, C. Baumgartner, E. Neufeld, M.C. Gosselin, D. Payne, A. Klingenbock and N. Kuster, IT’IS Database for thermal and electromagnetic parameters of biological tissues, Version 3.0, 2015. www.itis.ethz.ch/database