Making it Work: Manufacturing Technology
Once a functional product design has demonstrated the ability to meet the technical and market needs, developers must turn their attention to manufacturing the product with the required degree of precision, reliability and cost.
Simulation of all or part of the manufacturing process can be both informative as well as lead to quantifiable actions. For example, if thermal processes are used during fabrication does the local heat flow affect critical components? Are residual stresses developed that affect product performance and operating lifetime? Does the selected manufacturing process lead to material failure? Simulation of the manufacturing technology can then be used to modify the set up and operation of the manufacturing process to avoid any potentially catastrophic problems.
Among the typical manufacturing processes that can be simulated are:
-
Material fabrication
- Casting
- Injection molding
- Additive manufacturing
- Powder sintering
- Composite fabrication
-
Joining technology
- Thermal welding
- Friction welding
- Adhesive bonding
-
Product shaping
- Metal forming
- Cutting
- Rolling
- Extrusion
- Forging
-
Heat treatment
- Material property development
- Relief of residual stresses
- Surface conditioning
- Distortion
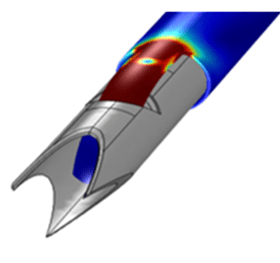
As an example of the use of PCM&S for the manufacture of medical devices, consider the manufacture of a tissue cutting tool using metal forming operations. As part of the manufacturing process the cutting component has to be formed to a specific radius without fracturing the cutter while simultaneously storing sufficient elastic energy to ensure rapid penetration through the selected tissue mass.
Figure 4.1: Stress distribution due to forming.
Simulation of the forming operation can address a wide range of issues such as the design of the forming tools, material selection, forming load evaluation, friction effects, forming stresses, material spring-back, etc.
In addition to simulating the response for a single output parameter, e.g, temperature, stress, flow velocity etc, one of the powerful aspects of simulation is the ability to isolate individual parameters that cannot be separated in experimentally based approaches and identify their significance for the manufacturing technology and product performance. By integrating the results of simulations with statistically significant systematic changes in input parameters, it is possible to identify what level of variance in device operation or design will lead to conditions where the device does not function correctly or the manufacturing technology would be expected to operate outside acceptable limits.
Further, information of this type can lead to identification of conditions where it may not be possible to maintain the required level of tolerance with the proposed manufacturing technology or can identify certain components that require monitoring to a specific level of accuracy to maintain product performance. When simulations are interfaced with commonly used statistical evaluation methodologies a powerful bridge is created that links product design, manufacturing technology and quality control.