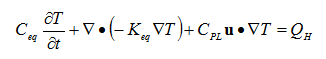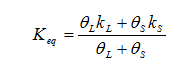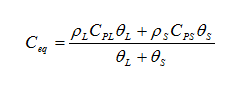Thermal-Ceramic Matrix Composites
In addition to fluid flow and chemical reaction, the thermal response during infiltration of liquid is important to include in any analysis of the RMI process. For CMCs of interest for aerospace use the liquid is molten Silicon with a melting temperature of approximately 1687K, and during infiltration this thermal energy must be dissipated. In addition, heat is also generated from two other sources: first, latent heat of fusion on transition from liquid to solid and secondly, heat of reaction as the molten silicon reacts with the carbon preform to form silicon carbide. All the thermal effects occur simultaneously with the liquid infiltration and chemical reaction.
Thermal effects due to the temperature of the liquid and the phase change form liquid to solid can be easily incorporated into the heat transfer calculations. Heat transfer from the reaction can be calculated using energy balance equations for a porous media:
Volume averaging is used to account for the liquid and solid phases,
giving an equivalent thermal conductivity and
heat capacity of porous media as:
where the subscript “L” refers to the liquid phase and the subscript “S” refers to the solid perform.
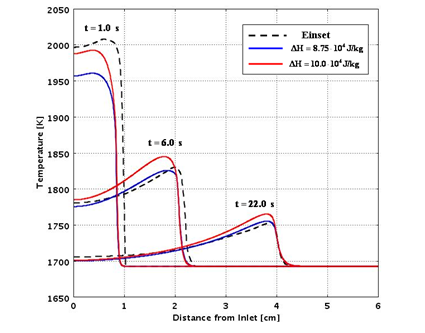
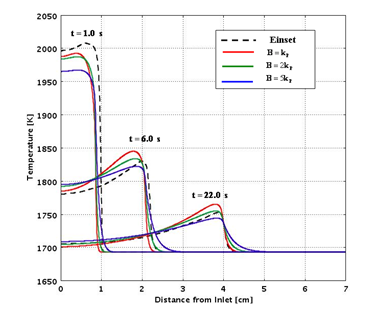
Figures 7 and 8 compare the predictions from the analytical routines with experimental data for a range of assumed values of the heat of reaction and thermal conductivity.
The next blog in this series will consider the development of residual stress and distortion in the CMC arising from phase changes and thermal mismatch. We welcome sharing of this information with your colleagues and coworkers.