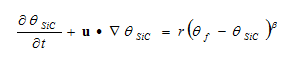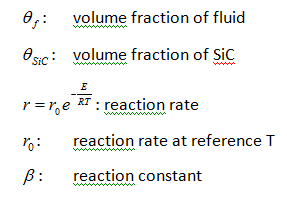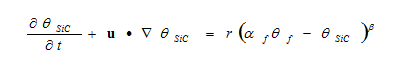Reaction-Ceramic Matrix Composites
In the last Blog we looked at analyzing fluid flow into a porous medium, in the manufacture of CMCs using the RMI process simultaneously with the fluid infiltration a reaction takes place between the infiltrating molten liquid and the porous preform. Thus the second step in developing a computational analysis of the RMI process for producing CMCs is to integrate the reaction behavior between the infiltrating liquid silicon and the porous carbon preform.
The reaction kinetics of the SiC formation are calculated using a general reaction kinetics equation with constants for the Si+C reaction:
Where:
The volume fraction of Si is obtained as:
The mass balance assumes that reaction proceeds to full completion and all Si transforms into SiC. To account for the effect of reaction termination, the mass balance can be modified as:
Where
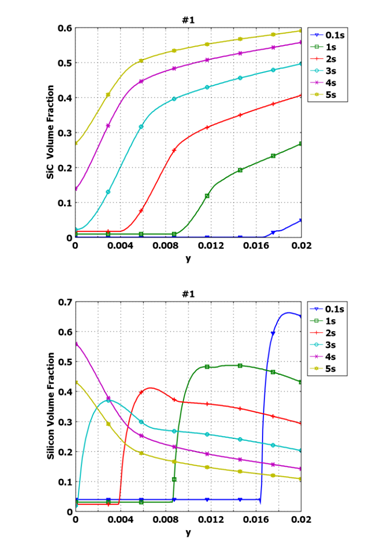
Integration of this approach in the model allows the distribution of SiC and Si species to be predicted as a function of both spatial location and time into the infiltration (Figure 6). Convection of the moving fluid and the reaction rate define the distribution of both species. The sum of the volume fractions of Si and SiC equals the total volume fraction of fluid.
Figure 6. Distribution of (a) SiC and (b) Si volume fractions along x=0
The next blog in this series will consider the issue of heat transfer associated with infiltration of molten silicon, reaction between the liquid silicon and the carbon preform, and heat evolved on solidification.