Proof-of-Concept: In the Beginning There Was…
Innovative product concepts and technologies invariably need to provide some level of Proof of Concept before an organization or agency will commit significant investment in money and/or resources to support continued development and commercialization. PCM&S is being increasingly used during the proof-of-concept phase to demonstrate technical feasibility, identify critical technical blocks and define the critical path for future success.
Consider the development of technology for the treatment of benign or malignant tumors: it is important to ablate undesirable tissue in a controlled and focused manner without affecting adjacent healthy tissue. Over the years, a number of minimally invasive techniques have been developed to selectively destroy tumors as an alternative to more invasive surgery. Each technique has specific advantages and disadvantages depending on the nature of the tumor and its location. A brief summary of currently available approaches is given below:
- Chemical ablation: chemical agents are injected into the undesirable tissue. Unfortunately, the affected area cannot be controlled because of the local blood flow and transport of the chemical species beyond the targeted tumor.
- Thermal ablation:
- Cryosurgery: a low temperature minimally invasive technique in which tissue is frozen on contact with a cryogenically cooled probe inserted into the undesirable tissue.
- Focused ultrasound: tissue is heated to coagulation using high-intensity ultrasound beams focused on the undesirable tissue.
- Radiofrequency (RF) ablation: an active electrode is introduced into the undesirable area and a high frequency alternating current is used to heat the tissue to coagulation.
- Interstitial laser coagulation: tumors are slowly heated to temperatures exceeding the threshold of protein denaturation using low power lasers delivered through optical fibers.
- Irreversible electroporation: a well-defined radiofrequency pulsed electric field irreversibly opens the cell membrane, thermal damage associated with the electric field distribution may also be superimposed, and tissue necrosis ensues.
High temperature thermal therapies have the advantage of ease of application but the disadvantage that the extent of the treated area is difficult to control because blood circulation strongly affects the temperature field developed in the tissue.
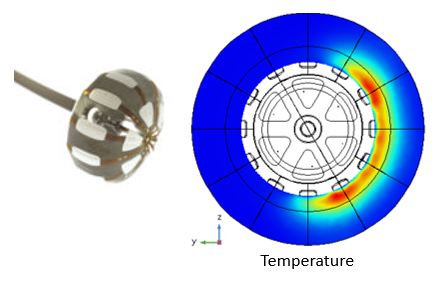
As developers try to minimize the spread of thermal or electroporation damage beyond the target volumes, they are turning to increasingly complex control systems and device designs. Consider the case of a balloon catheterization technology, Figure 2.1.
Figure 2.1: Simulation of temperature distribution associated with selected excitation.
Here, controlled local electrical and thermal transient histories of selected tissue volumes can be developed by simultaneously selecting patches that can be independently heated and cooled. Heating of selected patches is achieved by electrically exciting conductive patches while simultaneous cooling specified volumes by controlled fluid flow. This problem represents a highly coupled electrical-thermal-fluid multiphysics problem of the type that could not realistically be addressed without simulation. The large number of potential combinations of heated and cooled patches available with this approach is such that testing the large number of possible combinations would be impossible using conventional testing approaches.
To provide demonstration of the effectiveness of the technology to produce tissue necrosis with achievable operating conditions, a coupled electrical-thermal-fluid multiphysics simulation can be integrated with available mathematical methods for calculating tissue necrosis. There are a number of mathematical methods for calculating when cell death occurs due to thermal exposure. Among the most recognized are:
- A damage integral based on the Arrhenius equation provides the most general formulation.
- An Arrhenius rate approach in which the Cumulative Equivalent Minutes (CEM) at a specified temperature is calculated; for the case of tissue the temperature is usually taken as 43° The amount of time to produce the required damage for the tissue of interest is measured and used for comparison.
- A threshold temperature method is used when hyperthermic conditions are maintained for a short period of time. A target temperature is specified which, once exceeded, causes tissue necrosis. This binary approach is preferred for its simplicity, but may not provide a sufficient level of differentiation in regions of high thermal gradients.
Simulation provides a useful tool for assessing the temperature-time history of tissue subjected to a heat source; the calculations of tissue necrosis can be done directly based on the simulation output.
Through the use of multiphysics simulations, a large number of potential combinations of heating/cooling can be simulated to demonstrate the ability to control local temperature distribution in tissue, and thus tissue necrosis, as well as provide a platform for the development of patient specific treatment protocols based on an individual patient’s condition.