Medical device designers are responsible for many of the life-saving and life-enhancing therapies relied on by patients worldwide. From pacemakers to ablation tools to imaging devices, much of our current medical treatment would be impossible without the dedication of these engineers. But with the push to innovate comes a consistent pressure to develop new therapies in a competitive market while simultaneously meeting exacting safety standards, obtaining regulatory approval and reducing the time and cost for increasingly complex technology to reach the patient.
Within the past 15 years, requirements by regulatory bodies, coupled with the realities of an extremely competitive marketplace, have encouraged the incorporation of simulation within many stages of medical device design and development. In fact, the FDA represents one of the primary driving forces for expanding simulations in medical device design. Simulations solve the differential equations associated with the multiple physics through which medical devices operate, and predict the safety and performance of a device prior to physical prototyping and subsequent in vitro or in vivo testing. It’s a natural fit–putting the power of increasingly versatile and nimble computational simulation technology behind the design, development and implementation of complex, life-saving medical device technology.
Proof of Concept
Before the development of new medical device technology can begin in earnest, a funding agency or organization requires well-documented evidence showing that the project is feasible and the device will work as intended–better known as Proof of Concept. Computational simulation is increasingly used during the proof of concept phase to demonstrate technical feasibility, identify critical roadblocks and define the most likely path for future success.
Using simulation, developers can build predictive models to calculate the behavior of a device under a wide range of conditions. Adjustments can be made quickly to assess the response of different designs and operating conditions, allowing many scenarios to be investigated in a relatively short period of time. These simulations can provide invaluable insight into how a device might react under both ideal and “worst case scenario” conditions, preventing potential design problems, improving FDA approval packages, and saving capital outlay on physical prototyping and testing that’s better spent later in the design process.
Pushing the Envelope
Computational simulation provides an alternative approach to examining the limitations of initial design concepts and exploring the use of novel technology without the need for (and expense of) physical prototype development and testing. It also allows for a level of exploration and error-proofing that would simply not be possible in real world experiments.
Consider the development of ablation technology–it is important to ablate undesirable tissue in a controlled and focused manner without affecting adjacent healthy tissue. Over the years, a number of minimally invasive techniques have been developed to selectively destroy undesirable tissue as an alternative to more invasive surgery.
One such approach is the incitement of targeted tissue necrosis using a balloon catheter. Here, controlled local electrical and thermal transient histories of selected tissue volumes can be developed by simultaneously selecting patches that can be independently heated and cooled. Heating of selected patches is achieved by electrically exciting conductive patches while simultaneously cooling specified volumes by controlled fluid flow. This design objective represents a highly coupled electrical-thermal-fluid multiphysics problem of the sort that could not realistically be addressed without simulation. The high number of potential combinations of heated and cooled patches available with this method would make it impossible to investigate all possible scenarios using conventional testing approaches.
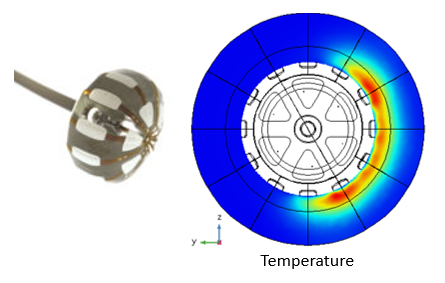
Using multiphysics simulations, an enormous number of potential combinations of heating/cooling can be simulated to demonstrate the ability to control local temperature distribution in tissue, and thus tissue necrosis, as well as provide a platform for the development of patient-specific treatment protocols based on an individual patient’s condition.
Reducing Time to Market
Finalizing product design is one of the most important stages in the development of a successful medical device. A flawed design may lead to a device being unsafe or simply ineffective. Traditionally, assessment of the safety, reliability and effectiveness of medical devices has relied on laboratory-based in vitro testing of physical prototypes and subsequent in vivo testing in animals and/or humans.
While physical testing can provide valuable information, it is slow, expensive and can only examine a limited number of variables. In vivo testing presents further difficulties in measuring any device performance–the inability to examine variations quickly and systematically, and ethical considerations associated with conducting extensive in vivo tests. Simulation can play an important role in the efficient development of safe and effective product designs, while reducing the time spent on physical prototyping/testing, and thus, time to market.
Take the case of a device to provide controlled drug delivery to the lungs. To efficiently pass through the respiratory tract and reach the lungs, aerosol droplets must contain a specific size distribution and provide a sufficient dose to be clinically effective. To efficiently enter the respiratory tract, the aerosol must be integrated with an inhaler mask that can ensure controlled entry to the lungs. Design of the inhaler mask must work for a range of breathing conditions and physical positions, while simultaneously ensuring passage of maximum doses of critical species into the lungs.
Simulations of the multiphase fluid flow behavior of the combination of droplets and gas provide an understanding of the multiphase flow within the mask to ensure delivery of the vapor to the respiratory tract. The simulation investigates the flow behavior for a range of breathing modes, i.e., combinations of mouth and nose, and physical positions of the head and respiratory tract. The simulations can be extended to investigate the influence of alternative mask designs and operating conditions to provide optimization of the flow characteristics with effective droplet transport to the lungs. In this way, the most effective design and operating conditions can be identified without the need to build and test a range of physical prototypes.
Ensuring Patient Safety
As discussed earlier, many medical devices (such as ablation tools) use radio frequency to create targeted heating and thus tissue necrosis. The benefits of simulation in the development of these devices are numerous. But they also extend to the development of implantable devices that may heat accidentally when exposed to electromagnetic radiation, such as that created by an MRI.
For devices with potential exposure to an MRI field, any passive implanted device must provide MRI safety labeling; there are a few exceptions, but the requirement for safety labeling is expanding, not shrinking. At the moment the major focus of the MRI safety requirement is centered on vascular devices, orthopedic implants and gastrointestinal devices.
In the past, devices such as orthopedic hip implants were designed for function first, then tested for MRI compatibility towards the end of the design cycle, often resulting in expensive and time-consuming redesigns if the implant failed the test. But the increasing adoption of simulation by device designers over the past two decades has allowed for “MRI-safe” design considerations to be integrated with structural design early on.
Simulations can be used to accurately predict the interaction between the electromagnetic field developed by the radio frequency (RF) coil of an MRI machine and an implanted medical device. They can be used to identify configurations of implanted devices that provide the highest temperature rise such that physical testing is then performed around that peak. And, because the thermal response of a device can be simulated, the effect of the body’s ability to dissipate heat can also be included, as can the effect of the body’s structure and its associated inhomogeneities.
Increasing Insight
Many industries are now experiencing the benefits of simulation-driven product development. Products that utilize complex physics are a natural fit for simulation, with the simulations helping provide an accurate virtual prototyping of a product’s design. Few products directly impact human health and longevity to the degree that medical devices do, and many medical devices are inherently multiphysics in nature, operating through multiple physical phenomena interacting with the human body.
The FDA is actively encouraging the use of simulation to support the development and use of medical products and technology for public health benefits, and as aspects of in vitro and in vivo trials begin to be replaced by in silico trials, computational simulation will become an evermore critical component in the development of new devices and patient therapies.
With simulation’s increased insight into the operation and limitations of medical device technology, a greater understanding of the complex interaction of medical device technology with human anatomy and physiology is being obtained, thus limiting potential risk to the public. Additionally, simulation promotes the safe and effective development of innovative medical technology while simultaneously reducing time to market, cutting development costs, and increasing overall insight and product knowledge.
Kyle C. Koppenhoefer is President of AltaSim Technologies. He has extensive experience in a wide range of computational mechanics applied to the medical products, automotive, petrochemical and defense industries.
