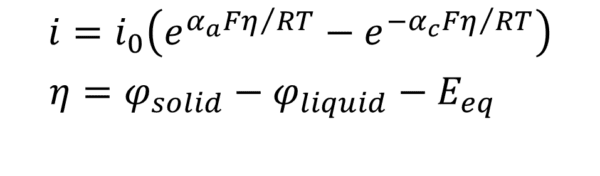The human body is a marvel of biological engineering, and one of its natural processes—sweating—plays a vital role in regulating body temperature. But human sweat, this seemingly innocuous liquid, is more than just water; it’s a complex solution of minerals, lactate, and urea, with sodium chloride being a major component. When sweat interacts with the electrical components of electronic devices, it can induce corrosion, dissolving metal or forming an oxide layer on the metal’s surface. Corrosion weakens the metal and disrupts electrical connections, leading to device failure.
Corrosion is due to the oxidation of metal atoms on anodic surfaces, and the corrosion process has three components:
- Reactions at anodic and cathodic metal surfaces that involve the transfer of electrons
- A pathway for the electrons to flow from the anode to the cathode
- Transport of ionic species through the electrolyte between anodic and cathodic surfaces
To combat the corrosive effects of sweat on electrical components, engineers choose materials with higher corrosion resistance, apply a protective coating, and design devices with minimal metal exposure.
Minimize Corrosion with Electrochemical Simulations
Engineers use electrochemical simulations to assist in the process of developing device designs that minimize corrosion. Electrochemical simulations use two fundamental approaches to describing the physics of the anodic and cathodic surfaces: polarization curves and reaction kinetics.
Polarization Curves
A polarization curve specifies the current density as a function of the electrode potential (voltage) during an electrochemical process. This approach uses experimental measurements of the current-voltage relationship for the specific electrode/electrolyte combination of interest. Polarization curves can provide many insights into the electrochemical behavior of a certain electrode material in a particular electrolyte, including the equilibrium potential and various aspects of anodic and cathodic behavior. These curves are relatively straightforward to measure experimentally and to use in numerical simulations, but they do have limitations:
-
-
- At a given electrode potential, the measured current may result from multiple electrochemical reactions and the contribution of each reaction cannot be differentiated solely based on the polarization data.
- A polarization curve accurately represents the electrochemical behavior only for the conditions it was measured under. Polarization behavior can be very sensitive to changes in the electrolyte’s composition or the electrode surface’s condition.
-
Nevertheless, polarization curves can provide useful predictions of corrosion behavior in many applications. When used in simulation, appropriate polarization curves are applied to each electroactive material in contact with the electrolyte, and the resulting current distributions and electrode potentials are calculated for the specified conditions. Corrosion may occur at locations where the electrode surfaces are anodic, and the rate of material dissolution at each location can be calculated by relating the local anodic current to the underlying oxidation reaction.
Reaction Kinetics
Corrosion involves at least one anodic and one cathodic electrochemical reaction. Models that explicitly include rate expressions for each reaction provide a more detailed description of corrosion processes than can be obtained using polarization curves. Every electrochemical reaction involves at least one electron transfer and proceeds at a rate dependent upon the local overpotential. The relationship between the overpotential and the reaction current is commonly described using the Butler-Volmer equation, which can take the following form:
where:
-
-
- i is the local current density
- i0 is the exchange current density
- αa is the anodic charge transfer coefficient
- αc is the cathodic charge transfer coefficient
- F is the Faraday constant
- η is the overpotential
- R is the universal gas constant
- T is the absolute temperature
- φsolid is the electrode potential at the electrode/electrolyte interface
- φliquid is the electrolyte potential at the electrode/electrolyte interface
- Eeq is the equilibrium potential
-
When the reaction kinetics are included in a corrosion simulation, the concentration-dependence of these reactions is also typically included, necessitating the modeling of species transport in the electrolyte. The resulting model is more complex than a model using the polarization curve approach, but a kinetic approach can provide much greater detail and can capture the influence of many factors on the corrosion process. While this approach has significant advantages, it is more computationally expensive than the polarization curve approach, and the accurate determination of reaction rate expressions can be challenging for complex systems.
Both polarization curves and reaction kinetics provide valuable insights into electrochemical behavior, and the preferred approach will depend upon the application. Engineers can leverage these tools to optimize electrochemical processes and understand corrosion mechanisms. Whether using polarization curves or reaction kinetics, simulation provides an excellent method of solving electrochemical challenges.